Learn types of colloids: lyophilic, lyophobic & association. Easy definitions, examples & diagrams for students, GPAT, and B.Pharm exams.
Introduction
Colloids are mixtures where one substance is evenly dispersed in another at a microscopic level. Based on the interaction between the dispersion medium and dispersed phase, colloids are classified into three major types:
- Lyophilic colloids
- Lyophobic colloids
- Association colloids
1. Lyophilic Colloids (Solvent-Loving Colloids)
Definition:
The word “lyophilic” comes from ‘lyo’ (liquid) and ‘philic’ (loving). These colloids have a strong affinity between the dispersed phase and the dispersion medium.
Key Features:
- Thermodynamically stable
- Easily prepared by simple mixing
- Reversible in nature (can be reconstituted by adding the medium again)
- Also called intrinsic colloids
Types Based on Solvent:
- Hydrophilic colloids: Water is the dispersion medium (e.g., starch, gelatin)
- Lipophilic colloids: Non-aqueous solvents are used (e.g., rubber in benzene)
Examples:
- Starch
- Gelatin
- Rubber
- Proteins
2. Lyophobic Colloids (Solvent-Hating Colloids)
Definition:
“Lyophobic” means ‘liquid-hating’. These colloids have little or no affinity for the dispersion medium. Hence, they are difficult to form and unstable.
Key Features:
- Thermodynamically unstable
- Require stabilizing agents
- Cannot be easily reconstituted
- Also called extrinsic colloids
Examples:
- Sols of metals like gold, silver
- Sols of metal hydroxides (e.g., Fe(OH)₃, Al(OH)₃)
Methods of Preparation:
1. Dispersion Methods:
These methods break down large particles into colloidal size.
- Ultrasonication
- Electric arc method
- Grinding & milling
- Colloid mills
- Peptization (breaking aggregates using a peptizing agent)
2. Condensation Methods:
In these methods, small molecules combine to form colloidal particles by chemical reactions like oxidation, reduction, hydrolysis, and double decomposition.
3. Association Colloids (Micelles)
Definition:
Also known as amphiphilic colloids, these colloids behave as electrolytes at low concentrations. However, at higher concentrations, they aggregate to form structures known as micelles.
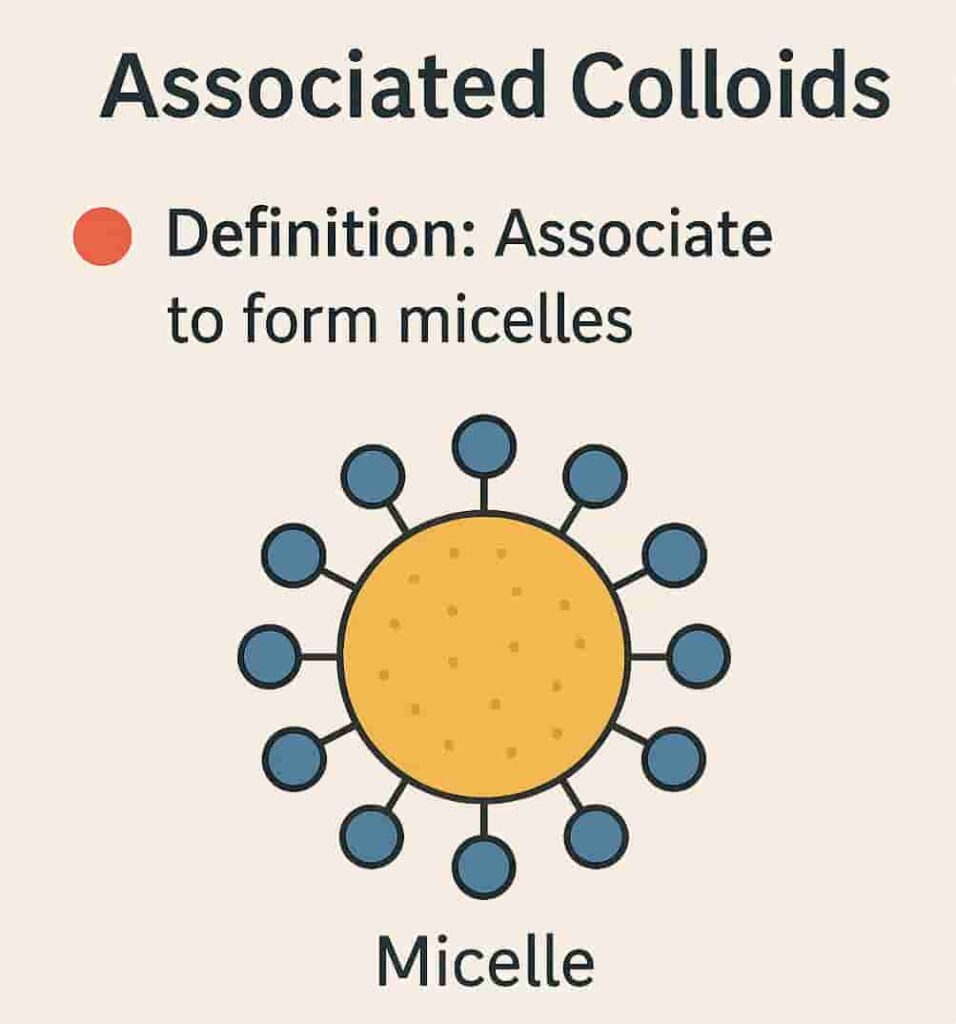
Micelle Structure:
- Hydrophilic “head”: Water-attracting
- Hydrophobic “tail”: Water-repelling
Types of Micelles:
- Spherical micelles
- Cylindrical micelles
- Inverse micelles
Critical Micelle Concentration (CMC):
This is the minimum concentration of surfactants required to start forming micelles.
- Below CMC: Molecules stay at the surface (air–water interface).
- At/Above CMC: Micelles form in the bulk phase, reducing system free energy.
Summary Table
| Type | Affinity with Medium | Stability | Reversibility | Example |
|---|---|---|---|---|
| Lyophilic Colloids | High (Loving) | Stable | Reversible | Starch, Gelatin, Rubber |
| Lyophobic Colloids | Low (Hating) | Unstable | Irreversible | Gold sol, Fe(OH)₃ sol |
| Association Colloids | Medium (Variable) | Conditional | Conditional | Soaps, Detergents (Micelles) |
Detailed Explanation ( in Hindi ) : Classification of Colloids
Introduction – Colloids kya hote hain?
Colloid ek aisa mixture hota hai jisme ek substance (dispersed phase) dusre substance (dispersion medium) mein evenly spread hota hai, lekin itna chhota hota hai ki microscope se hi dikhta hai.
Ye true solution se bada hota hai aur suspension se chhota.
● Example: Doodh — ismein fat particles water mein colloidal form mein hote hain.
👉 Ab colloids ko 3 types mein divide kiya jaata hai, based on unka medium ke saath interaction:
- Lyophilic colloids – Jo solvent se dosti karte hain.
- Lyophobic colloids – Jo solvent se dosti nahi karte.
- Association colloids – Jo micelle banaate hain (jaise soap).
1. Lyophilic Colloids – Solvent-Loving Colloids
Naam ka matlab:
- “Lyo” = Liquid (yaani solvent)
- “Philic” = Loving (pyaar karna) 👉 Matlab: Solvent ko pasand karte hain.
Kya hota hai?
Ye colloids mein dispersed phase aur dispersion medium ke beech strong attraction hoti hai.
Important Features:
| Feature | Explanation |
|---|---|
| 🔹 Stability | Highly stable — khud hi ban jaate hain. |
| 🔹 Reversible | Agar dry ho jaaye to phir se solvent daal ke wapas colloid ban sakta hai. |
| 🔹 Easy to prepare | Bas simple mixing se ban jaata hai. |
| 🔹 Thermodynamically stable | Energy-wise bhi ye stable hote hain. |
Types (Solvent ke base par):
- Hydrophilic colloid: Agar solvent paani ho. (e.g., starch in water)
- Lipophilic colloid: Agar solvent oil-type ya benzene ho. (e.g., rubber in benzene)
🌟 Examples:
- Starch
- Gelatin
- Rubber
- Proteins
2. Lyophobic Colloids – Solvent-Hating Colloids
Naam ka matlab:
- “Lyo” = Liquid
- “Phobic” = Darr ya Nafrat 👉 Matlab: Solvent se dosti nahi karte.
Kya hota hai?
Ismein dispersed phase aur medium ke beech weak attraction hoti hai, isliye ye unstable hote hain.
Important Features:
| Feature | Explanation |
|---|---|
| 🔹 Stability | Unstable — time ke saath settle ho jaate hain. |
| 🔹 Irreversible | Ek baar dry ho gaya, to phir wapas colloid nahi banega. |
| 🔹 Difficult to prepare | Normal mixing se nahi banta — special method chahiye. |
| 🔹 Thermodynamically unstable | Energy-wise unstable hote hain. |
Examples:
- Gold sol
- Silver sol
- Ferric hydroxide sol [Fe(OH)₃]
- Aluminium hydroxide sol [Al(OH)₃]
Preparation Methods (Important for Exams)
1. Dispersion Methods:
👉 Bade particles ko tod-tod ke chhote colloidal size mein laana.
- Ultrasonication – Sound waves se todna
- Electric arc method – High voltage arc se vapour banake sol banana
- Grinding/Milling – Mechanical tareeke se todna
- Colloid mill – High-speed rotating machinery
- Peptization – Aggregated particles ko peptizing agent daal ke disperse karna
2. Condensation Methods:
👉 Chhoti molecules ko chemical reaction se bada colloidal particle banana.
- Oxidation
- Reduction
- Hydrolysis
- Double decomposition
3. Association Colloids (Micelles)
Kya hota hai?
- Inhe Amphiphilic colloids bhi kehte hain.
- Ye normal ions ki tarah behave karte hain jab unka concentration kam hota hai.
- Jab concentration ek limit se zyada ho jaata hai, to ye micelles bana lete hain.
Micelle ka structure:
| Part | Nature |
|---|---|
| Head | Hydrophilic (water loving) |
| Tail | Hydrophobic (water hating) |
Ye tail-inside aur head-outside structure banata hai — taaki tail water se door rahe.
Types of Micelles:
- Spherical micelles – Round shape
- Cylindrical micelles – Long tube shape
- Inverse micelles – Head inside, tail outside (non-aqueous solvent mein)
📍 Critical Micelle Concentration (CMC)
👉 CMC wo minimum concentration hoti hai, jahan se micelles banana shuru hota hai.
| Concentration | Behavior |
|---|---|
| Below CMC | Surfactant molecules surface par rehte hain |
| At/Above CMC | Micelles ban jaate hain (bulk mein) |
📊 Summary Table (Yaad karne ke liye best)
| Type | Medium ke saath rishta | Stability | Reversible? | Examples |
|---|---|---|---|---|
| Lyophilic Colloid | Dosti (High affinity) | Stable | Haan | Starch, Gelatin, Rubber |
| Lyophobic Colloid | Dushmani (Low affinity) | Unstable | Nahi | Gold sol, Fe(OH)₃ sol |
| Association Colloid | Beech ka rishta | Conditional | Conditional | Soaps, Detergents (Micelles) |
Exam Tips (Pro Level)
- ❓ Differentiate between lyophilic and lyophobic colloids – 5-mark question ke liye table banake likho.
- ❓ Explain micelle formation with CMC – Diagram zaroor banao.
- ❓ Methods of preparation of lyophobic colloids – Peptization, ultrasonication yaad rakho.
Sources:
| Source | Contribution |
|---|---|
| CVS Subrahmanyam (Physical Pharmaceutics-II) | 80% – Classification, features |
| KD Tripathi, Remington | 10% – Terms like intrinsic/extrinsic |
| GPAT, RGUHS previous year papers | 10% – Question pattern, examples |
FOR NEXT
